Atoms
An introduction to Atoms
Name: Own Teacher
Email: info@ownteacher.com
Created At: 01-11-2023
Atoms are the fundamental building blocks of matter. They are the smallest units of an element that retain the properties of that element. Atoms consist of three primary subatomic particles:
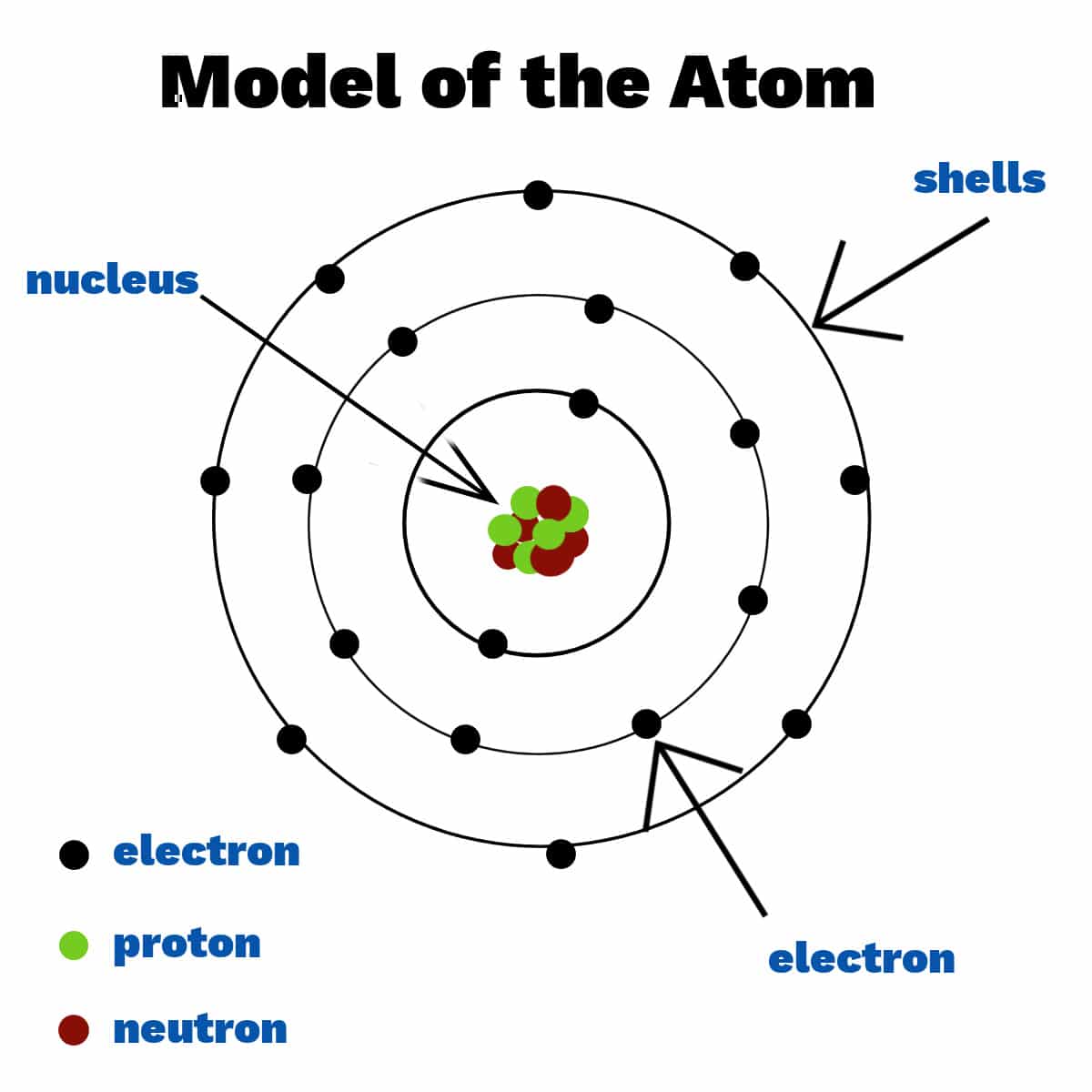
Protons: Positively charged particles found in the nucleus, the central core of the atom. The number of protons determines the element's identity and is known as the atomic number.
Neutrons: Particles with no electrical charge, also found in the nucleus. Neutrons contribute to the atom's mass and stability.
Electrons: Negatively charged particles that orbit the nucleus in electron shells. Electrons are involved in chemical reactions and bonding with other atoms.
Key Concepts:
Atomic Structure: Atoms have a central nucleus containing protons and neutrons, with electrons orbiting in various energy levels or shells.
Elements: Each element on the periodic table is defined by the number of protons in its atoms' nuclei.
Isotopes: Atoms of the same element with the same number of protons but different numbers of neutrons. Isotopes have slightly varying properties.
Atomic Mass: The mass of an atom is primarily determined by the combined mass of protons and neutrons. Electrons contribute very little to an atom's mass.
Electron Configuration: Electrons are arranged in specific energy levels or orbitals, with rules governing their distribution.
Chemical Bonding: Atoms interact by forming chemical bonds to create molecules or compounds. Bonds can be ionic (electron transfer) or covalent (electron sharing).
Quantum Mechanics: Understanding atomic behavior involves quantum mechanics, a branch of physics dealing with the behavior of particles at atomic and subatomic scales.
Atomic Spectroscopy: The study of how atoms interact with electromagnetic radiation, often used for elemental analysis and identifying substances.
Applications: Atomic research has broad applications in chemistry, physics, materials science, and technology, including nuclear energy, semiconductor technology, and nanotechnology.
Atoms are the foundation of chemistry, and their behavior and interactions dictate the properties of matter. The study of atoms has led to significant advancements in our understanding of the physical world and has practical applications across various scientific and technological fields.
What is an atom?
An atom is the basic building block of all matter and chemistry. It is the smallest unit of a chemical element that retains the chemical properties of that element. Atoms can combine with other atoms to form molecules but cannot be divided into smaller parts by ordinary chemical processes .
Are all atoms the same size?
No, atoms are not all the same size. The size of an atom can vary depending on the element and its atomic structure. Atomic dimensions are thousands of times smaller than the wavelengths of light, so they cannot be viewed using an optical microscope. However, individual atoms can be observed using a scanning tunneling microscope.
What does the mass of an atom consist of?
The mass of an atom consists of its subatomic particles: protons, neutrons, and electrons. Protons and neutrons are located in the nucleus of the atom, while electrons orbit around the nucleus. Protons have a positive charge, neutrons have no charge (they are neutral), and electrons have a negative charge. The mass of an atom is primarily determined by the combined mass of its protons and neutrons, while the electrons contribute very little to the overall mass of the atom .
How is the atomic number of an atom defined?
The atomic number of an atom is defined as the number of protons in the nucleus of the atom. It is a unique identifier for each element on the periodic table. For example, hydrogen has an atomic number of 1 because it has one proton in its nucleus, while carbon has an atomic number of 6 because it has six protons in its nucleus , The atomic number determines the chemical properties and identity of an element.
Comment List
Leave a Comment.