Mass Spectrometry
An introduction to Mass Spectrometry
Name: Own Teacher
Email: info@ownteacher.com
Created At: 28-10-2023
Mass spectrometry (MS) is an analytical technique used to identify and characterize the chemical composition of molecules based on their mass-to-charge ratio (m/z). Here's a full explanation of mass spectrometry:
Principle:
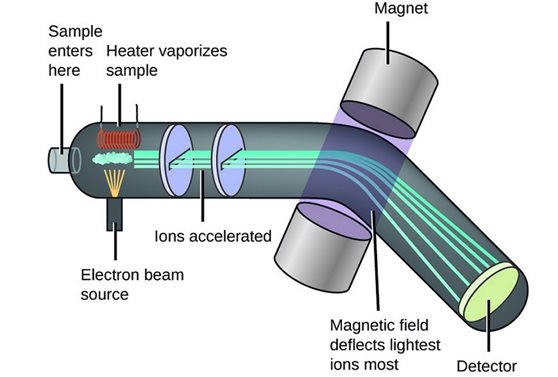
Ionization: A sample is first ionized, typically using techniques like electron impact, electrospray, or laser ablation. This process converts neutral atoms or molecules into ions by adding or removing electrons.
Mass Separation: The ions are then accelerated and passed through a mass analyzer. Common mass analyzers include quadrupoles, time-of-flight (TOF), magnetic sector, and ion trap analyzers. These analyzers separate ions based on their mass-to-charge ratio.
Detection: As ions exit the mass analyzer, they are detected. The detector measures the abundance of ions at different m/z values, generating a mass spectrum.
Components of a Mass Spectrum:
Molecular Ions: Molecules with an unaltered charge. They have an m/z value corresponding to the molecular weight.
Fragment Ions: Result from the fragmentation of molecular ions due to the ionization process.
Base Peak: The most intense peak in the mass spectrum, often the molecular ion or a major fragment.
Applications:
Qualitative Analysis: MS is used to identify the composition of compounds, analyze complex mixtures, and determine the structure of unknown substances. The mass spectrum provides information on the molecular weight, isotopic pattern, and fragment ions.
Quantitative Analysis: MS can quantify the concentration of specific compounds in a sample, commonly in fields like environmental analysis, pharmaceuticals, and clinical chemistry.
Proteomics and Metabolomics: MS is vital in studying proteins (proteomics) and metabolites (metabolomics). It identifies and quantifies biomolecules, aiding in biological research and disease diagnosis.
Forensic Analysis: MS is used in criminal investigations to analyze drugs, explosives, and trace evidence.
Environmental Monitoring: It helps detect pollutants and toxins in air, water, and soil.
Advantages:
- High sensitivity and selectivity.
- Can analyze a wide range of compounds, from small molecules to large biomolecules.
- Provides structural information.
- Allows for isotopic analysis.
Limitations:
- Expensive equipment and maintenance.
- Complex data analysis.
- May require sample preparation.
- Destructive technique for some samples.
In summary, mass spectrometry is a versatile analytical technique that plays a crucial role in various scientific disciplines, offering a powerful means for identifying, characterizing, and quantifying a wide range of compounds and molecules.
Comment List
Leave a Comment.