Mean free path in Chemistry
An introduction to Mean free path in Chemistry
Name: Own Teacher
Email: info@ownteacher.com
Created At: 06-11-2023
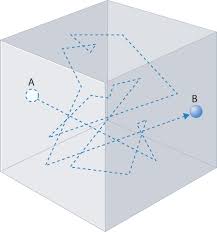
Mean free path is a fundamental concept in chemistry and physics, particularly in the study of gases. It refers to the average distance a particle, such as a gas molecule, travels between successive collisions with other particles in a gas or a fluid. This concept is crucial for understanding the behavior of gases on a molecular level and is a key component of kinetic theory.
Here's a more detailed explanation of mean free path:
Particle Collisions: In a gas or fluid, particles are in constant random motion. They collide with each other, and these collisions are responsible for various macroscopic properties of the gas, such as pressure, diffusion, and viscosity.
Average Distance: The mean free path is the statistical average of the distances traveled by a particle between these successive collisions. It represents the typical distance a particle covers before encountering another particle.
Gas Behavior: Mean free path is a crucial parameter in understanding gas behavior. In a gas with a high mean free path, particles are relatively far apart, and the gas behaves like an ideal gas. In contrast, in a gas with a low mean free path, particles are closely packed, and deviations from ideal gas behavior are more pronounced.
Factors Affecting Mean Free Path: The mean free path depends on several factors, including the size and shape of the gas molecules or particles, their velocity, and the gas pressure. Smaller molecules, higher velocities, and lower pressures tend to result in longer mean free paths.
Transport Phenomena: The concept of mean free path is essential in explaining various transport phenomena, such as heat conduction, diffusion, and viscosity. For example, the mean free path of gas molecules plays a role in determining the rate of diffusion and the thermal conductivity of the gas.
Knudsen Number: The Knudsen number, which is the ratio of the mean free path to a characteristic length scale of the system, is often used to determine the degree of rarefaction (the departure from the continuum fluid dynamics regime) in a gas flow.
In summary, the mean free path is a vital concept in chemistry and physics, especially in the study of gases. It provides insight into the behavior of gas molecules on a microscopic level and helps explain various macroscopic properties and phenomena related to gases and fluids.
Comment List
Leave a Comment.